Infrared Analysis
All instruments in the lion intoxilyzer® range operate on the principle of infrared absorption spectroscopy for the analysis of the breath specimen.
To understand the basic theory of infrared light absorption, consider what happens when you look at an ordinary light bulb. This bulb emits light – visible light – to which your human eye is sensitive. So visible light, falling on your eye, causes a signal to be sent from your eye to your brain. This signal, in effect, tells you how bright the light bulb is.
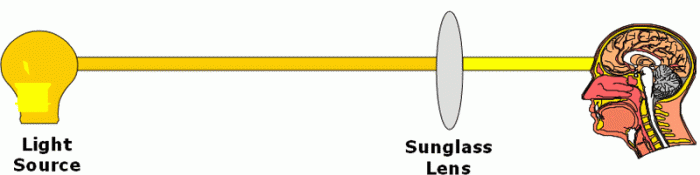
If you now put on a pair of sunglasses, the lens has the effect of absorbing some of the visible light passing from the bulb to your eye. This means that less light reaches the eye, which causes a change in the signal passing from the eye to the brain. The result of this change is that the bulb appears to be less bright.
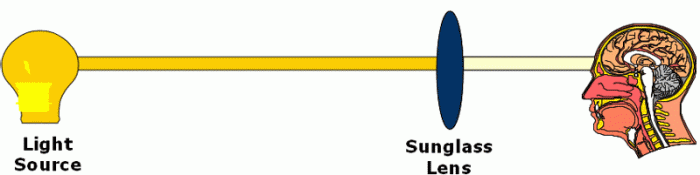
Now, just as the lens in a pair of sunglasses absorbs visible light, alcohol has the property of being able to absorb infrared light.
So, the lion intoxilyzer® 8000, for instance, consists of an infrared source (the bulb), attached to one end of a gas chamber (the lens) through which passes the breath. At the other end of this chamber is an infrared detector (the eye), which is connected to the measuring electronics and, eventually, to the computer section (the brain) of the instrument.
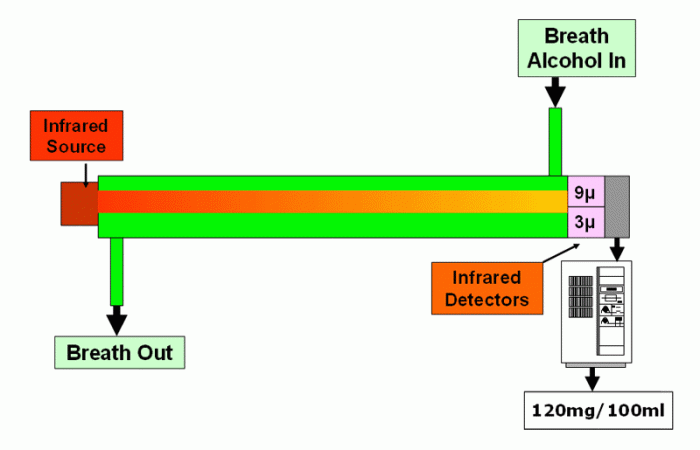
Starting with the gas chamber full of clean air, infrared light passing from the source flows through it and falls on the detector at the other end. If alcohol is now introduced into the chamber, this absorbs some of the light passing through that chamber, so less light falls on the detector. This change in light intensity is interpreted by the instrument in terms of the concentration of alcohol in that sample (ie how dark the lens is to infrared light).
The greater the concentration of alcohol in the breath sample, the more infrared light that is absorbed. This is known as Beer’s Law [from a scientist of that name, back in 1852].
