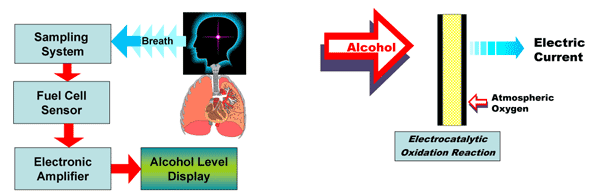
Fuel Cell Sensors for Breath Analysis
The analytical heart of all lion alcolmeter® range is the Lion fuel cell sensor. This device receives the breath sample, from which any alcohol that is present in it becomes instantly absorbed onto its working electrode: this electrode is made from a specially prepared platinum-black catalyst material.
On this electrode a spontaneous oxidation reaction of the alcohol takes place, which so results in the loss of electrons from its molecules. These flow around an external circuit to the counter electrode, where oxygen from the air undergoes a concurrent reduction, so receiving the electrons.
This transfer of electrons from the alcohol electrode to the oxygen electrode via an external circuit constitutes an electric current. The larger the number of alcohol molecules that were present in the sample – in other words, the greater its concentration – then the more electrons that are generated, and so the greater the electric current that flows.
So by measuring the electric current that flows from the alcohol electrode in the fuel cell, we can determine the concentration of alcohol that must have been present in the specimen of breath that was introduced into it.The Lion fuel cell has a high specificity to alcohols, and, although not totally ethanol-specific, it is very unlikely that the breath of a living human would ever contain any other chemical substance to which this sensor responds.
The Lion fuel cell has a high specificity to alcohols, and, although not totally ethanol-specific, it is very unlikely that the breath of a living human would ever contain any other chemical substance to which this sensor responds.
Finally, the Lion fuel cell operates at ambient temperature, and requires no external power source – apart from that to the electronic measuring circuit. This alcohol sensor also has a long working life, typically over five years.
